Description

Liquid Oral Plants Features
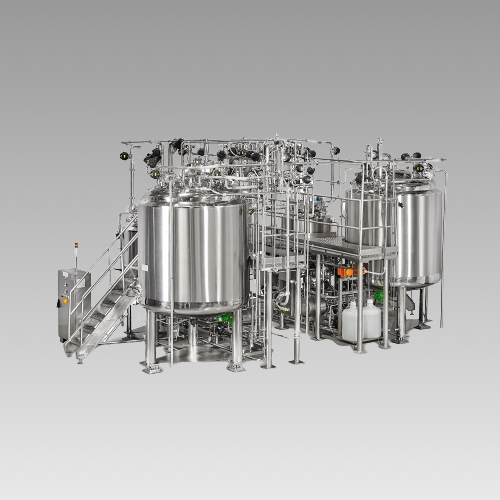
Liquid Oral Processing Plant for Syrups & Suspensions, as per the customers’ requirements to meet WHO, USFDA and MHRA norms. The equipments are manufactured as per cGMP guidelines and ASME standards. We are the leader in providing the latest technology in Liquid Processing Solutions in Semi-Auto or completely automated plant with SCADA SYSTEM.
- We can offer you the complete plant comprising:
- Design and detailed Engineering
- Equipments & Piping Layouting
- Sugar and Glucose Transfer System
- Sugar Syrup Preparation with efficient bottom entry agitation
- Agitated or Non-Agitated Storage Vessels for the Filling line with change over panels
- CIP & SIP System for cleaning & sanitization of the system
- SS Operational platform with Stair & Railing
- Sterile Piping System with Sterile Control Valves and most suitable instrumentation
- PLC based Automation System as per 21 CFR Part 11 (Optional)
- System Qualification Documents like DQ, FAT, IQ & OQ Protocols
- State-of-the-Art manufacturing under highly qualified workshop by skilled labour
- Efficient site teams for the erection and assistance in the validation of the process cycle.
- “CE” marked Equipments are offered with Third Party Inspection & All Electrical Control with ERTL Approval & Test
- Prompt After Sales Services
Specifications
- Crevice & Dead leg free design, Contact surfaces to less than 0.5 m RA finish from inside
- Complete drainability
- Contact Parts: MOC SS 316L
- Non-Contact MOC SS 304 finished to 0.8 m RA Matt
- Completely suitable for in-line CIP & Sanitization
- Product Sampling devices
- Food Grade Gaskets in compliance with FDA 21 CFR Part 177-2600
- Zero Dead-Leg Flush Bottom Diaphragm Valves
Applications
- Cough Syrups
- Oral Drops
- Expectorants
- Antacid Suspensions
Our Products
Download Brochure
Kindly fill in your details. All fields mandatory.
Product Enquiry Form
Trust Me! We are Engineers
We are committed to be your brain, eye and heart designing and bring the projects and products with our experience in the industry.