
SchedulePro
Intelligen Inc. developed highly customizable schedule tool for batch and semi-continuous processes. EMA Engineering partners and distributes Schedule Pro in Turkey. SchedulePro is a finite capacity scheduling (FCS) tool for batch and semi-continuous manufacturing processes.
- Production Planning and Scheduling
- Capacity Analysis
- Plant Debottlenecking
- Cycle Time Reduction
- Modeling and Design of Multi-Product Facilities
SchedulePro uses an intuitive recipe-oriented representation of a manufacturing process. It handles resources such as equipment, work areas, labor, materials, utilities and inventory capacity. It allows for interruptions in the availability of resources to account for shift schedules, holidays, and planned maintenance. SchedulePro is compatible with SuperPro Designer and can readily import its recipes.
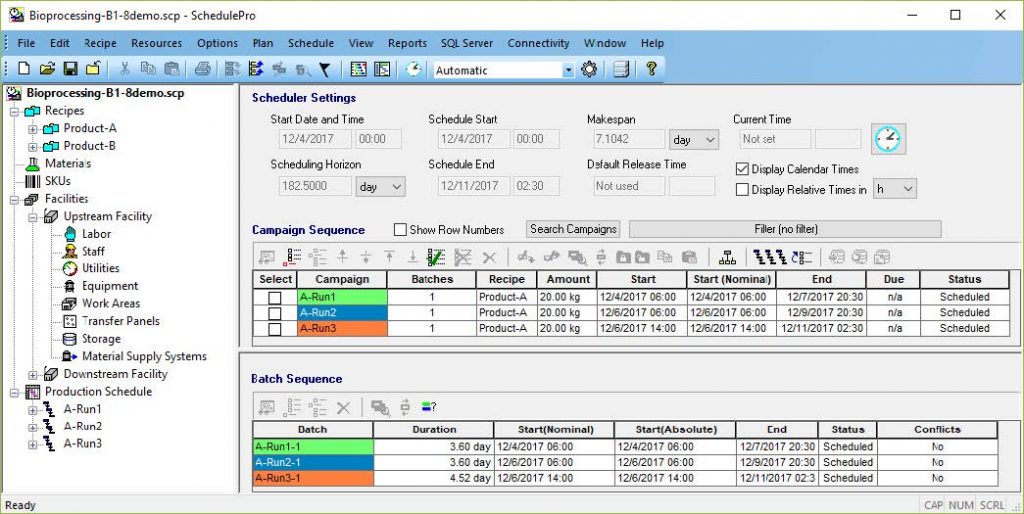
“SchedulePro uses an intuitive recipe-oriented representation of a manufacturing process. It handles resources such as equipment, work areas, labor, materials, utilities and inventory capacity.“
To download SchedulePro’s full manual (in pdf format), please click here. The posted zip file has a size of 8.5 MB. If you go through Chapter 4 (the tutorial), you will have a pretty good idea of the capabilities of the tool. To download the ReadMe files (in PDF format) for eight domain specific examples, please click here. Information on the contents of the examples follows:
- Bio-Processing: It focuses on modeling and scheduling of biopharmaceutical facilities.
- Ice-Cream: It explains how to optimize the scheduling of a facility that manufactures a wide variety of ice cream products.
- Pharma-Tablets: It deals with modeling and scheduling of a facility that manufacturers pharmaceutical tablets.
- Capacity-Analysis: It explains how to handle capacity analysis and long term planning.
- CMO-Planning: It explains how to handle production planning (i.e., high-level scheduling).
- Fill-Finish: It deals with scheduling and planning of a fill-finish facility.
- Polymer-Resins: It deals with scheduling of a polymer resin multi-product manufacturing facility.
- Yogurt: It deals with production of yogurt utilizing SKUs and SKU-Order-Templates.
And if you wish to download and test-drive the functional Evaluation version of SchedulePro, please click here.
SchedulePro is available for the MS Windows XP, Vista, 7 and 8 platforms. It requires a Pentium PC with at least one GB of RAM, and 100 MB of free hard disk space.
- Multi-product, multi-plant scheduling
- Compatibility with SuperPro Designer
- Handles plant down-time, weekend and holiday schedules
- Schedules around equipment and material constraints
- Handles labor and inventory constraints
- Tracks completion status of scheduled operations
- Extensive reporting and charting capabilities
- No programming required


- Discount is available for multiple licenses.
- Departmental, site and corporate licenses are available under very attractive terms.
- Educational licenses are available at substantial discounts.
- A FUNCTIONAL EVALUATION version is available to download (FREE OF CHARGE) or order for a small fee.


- Discount is available for multiple licenses.
- Departmental, site and corporate licenses are available under very attractive terms.
- Educational licenses are available at substantial discounts.
- A FUNCTIONAL EVALUATION version is available to download (FREE OF CHARGE) or order for a small fee.
- Design and Optimization of a Large Scale Biopharmaceutical Facility using Process Simulation and Scheduling Tools, Toumi A, Jürgens C, Jungo C, Maier B, Papavasileiou V, and Petrides D. , Pharmaceutical Engineering, March/April 2010 issue.
- The Role of Simulation and Scheduling Tools in the Development and Manufacturing of Active Pharmaceutical Ingredients, Petrides D, Koulouris A, Siletti C, Jimenez J and Lagonikos P., An improved version of this document will become available as a chapter of a new book on “Chemical Engineering in the Pharmaceutical Industry: From R&D to Manufactiuring” that will be published by John Wiley & Sons in 2010.
- The Role of Process Simulation in Pharmaceutical Process Development and Product Commercialization, Demetri P. Petrides, Alexandros Koulouris, and Pericles Lagonikos, Pharmaceutical Engineering. January/February 2002, Pp. 56-65. Click here to download a copy of this paper in PDF format.
- Throughput Analysis and Debottlenecking of Biomanufacturing Facilities – A Job for Process Simulators, Demetri Petrides, Alexandros Koulouris, and Charles Siletti, BioPharm. Volume 15, Numbre 8, August 2002, Pp. 28-64. Click here to download a copy of this paper in PDF format.
- Selection of Bioprocess Simulation Software for Industrial Applications, Shanklin, T., Roper, K., Yegneswaran, P.K., and Marten, M. Biotechnology and Bioengineering. Vol. 72, No. 4, February 20, 2001, pp 483-489. This article includes a comparison of SuperPro Designer with Bach Plus for modeling and evaluating integrated biochemical processes. It was published by researchers from Merck & Co. and UMBC (University of Maryland at Baltimore County).
- Batch Process Optimization via CAPD and Simulation, Alexandros Koulouris and Demetri P. Petrides, PROCESS Worldwide 2-2002, Pp. 38-40.
- Process Modeling Evaluates Feasibility of Water Recycling, Demetri Petrides, Alexandros Koulouris, and John Calandranis, Filtration+Separation, October 2001, Pp. 26-31. Click here to download an earlier version of this paper.
- The Role of Process Simulation in Evaluating Water Recycling Opportunities at a Semiconductor Fabrication Facility, Demetri Petrides, Charles Siletti, and John Calandranis, ULTRAPURE WATER, July/August 2002, Pp. 26-34. Click here to download an earlier version of this paper.
- Process and Economic Evaluation of the Extraction and Purification of Recombinant b-Glucuronidase from Transgenic Corn, R.L. Evangelista, Ann R. Kusnadi, John A. Howard, and Zivko L. Nikolov, Iowa State University and Prodigene, Inc., Biotechnology Progress, 1998, 14, Pp. 607-614.
- Process Simulation for Recombinant Protein Production: Cost Estimation and Sensitivity Analysis for Heparinase I Expressed in E. Coli. Steffen Ernst, Oscar A. Garro, Stefan Winkler, Ganesh Venkataraman, Robert Langer, Charles L. Cooney, and Ram Sasisekharan, Biotechnology and Bioengineering, Vol. 53, No. 6, March 20, 1997.
“EMA Engineering always provides cost effective, good quality engineered solutions.” For further information regarding product and services please contact with us.
Error: Contact form not found.
