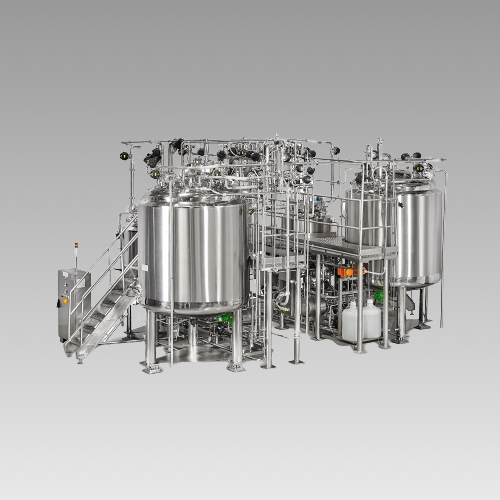
Description
Parenterals Plants (Large Volume)

Features
We offer you highly qualified technical services in every aspect to meet the highest criteria of sterility in minute design as well as all the required specifications with innovative and efficient process technology for Parenterals applications in complete compliance with WHO, USFDA and MHRA regulations. The equipments are designed as per ASME guidelines.
Our range of expertise starts from Designing, Developing, Manufacturing in strict cGMP compliance, carrying out FATs, supplying and supervising site erection with the execution of sterile piping and Automation System for the latest high quality LVP Process Plants with all the required qualification documents.
The Automation System is designed as per GAMP, 21 CFR Part 11 compliant (Optional)
- Detail Design & Engineering
- Automation
- Manufacturing
- Qualifications
- Installation Supervision
- Piping Systems
- CIP & SIP Systems
- Assistance in validation
- After Sales Services
Specifications
- Crevices and dead leg free contact surfaces, electropolished from inside
- 100% Drainability
- Completely suitable for CIP & SIP
- Aseptic Sampling facility
- Flush mounted Tank Bottom Diaphragm Valve
- Sterile Micron Filtration System
- Food Grade Gaskets in compliance with FDA 21 CFR Part 177-2600
- State-of-the-Art Bottom Entry Mixing System with Sterile Double Mechanical Seals OR Bottom Magnetic Stirrers
- Vacuum Powder Addition System
Applications
- Infusion Solution
- Electrolytes
- De-aeration
Our Products
Download Brochure
Kindly fill in your details. All fields mandatory.
Product Enquiry Form
Trust Me! We are Engineers
We are committed to be your brain, eye and heart designing and bring the projects and products with our experience in the industry.